News
Study Demonstrates vMap Localization for Hemodynamically Unstable VT
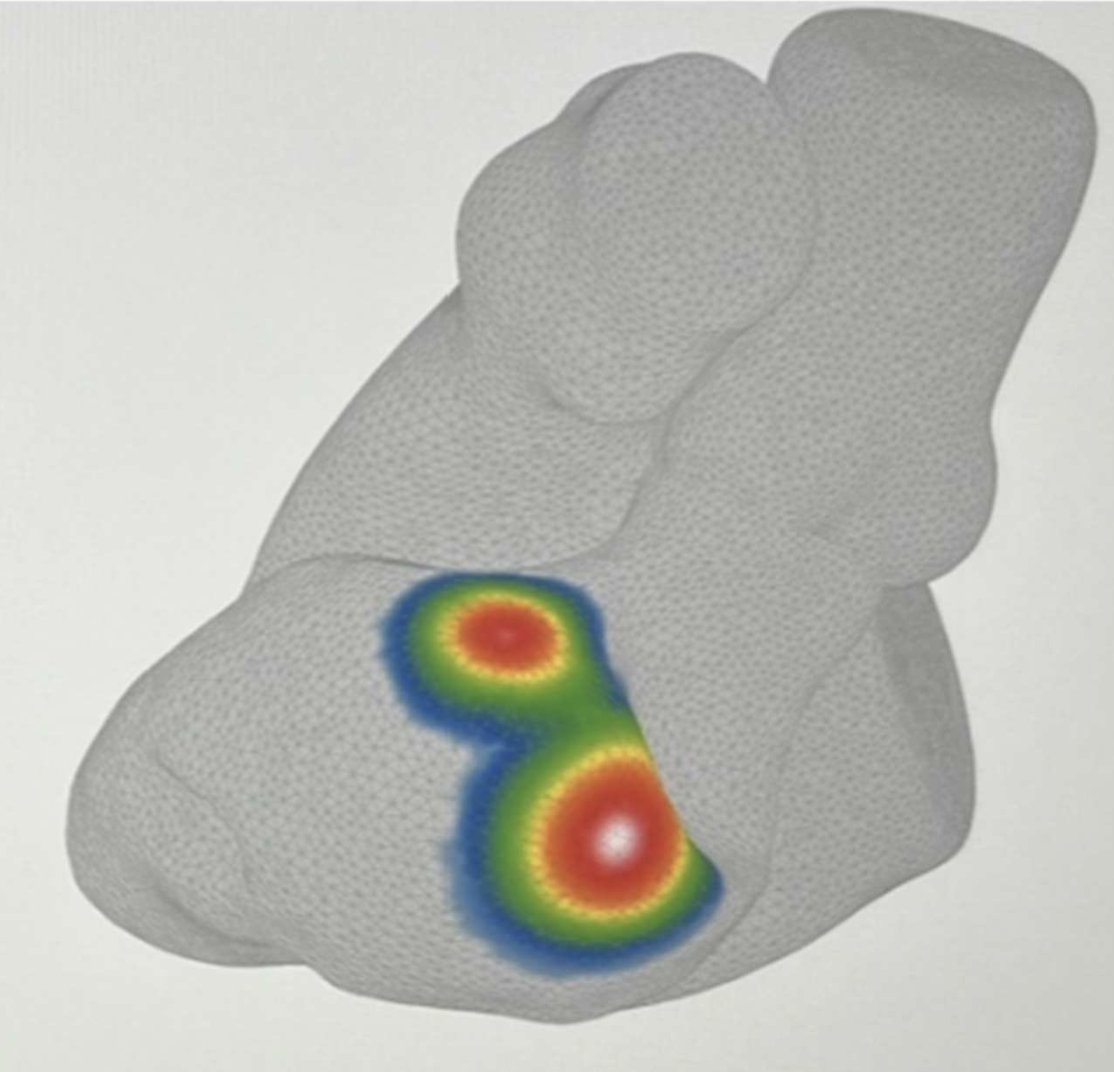
The Journal of Cardiovascular Electrophysiology today published a proof-of-concept study evaluating vMap’s approach to assisting localization of ventricular tachycardia (VT) exit sites in patients with hemodynamically unstable, scar-mediated VT.
The single-center study examined agreement between vMap analysis of standard 12-lead ECGs and substrate-based mapping performed in sinus rhythm, including pace-mapping and identification of channels of slow conduction within scar tissue - an approach that may be useful when activation mapping is limited by hemodynamic instability and scar complexity.
Across nine induced VT rhythms in four patients, vMap analysis showed agreement with the sites of successful ablation in cases where it was used. After ablation targeting those sites, the mapped VTs were not re-induced. At six months of follow-up, no patients experienced device therapy for recurrent VT or all-cause mortality.
Overall, the findings suggest that combining vMap analysis with established substrate-based mapping techniques may help support procedural planning in complex VT ablation cases. Further study in larger patient populations may help clarify clinical utility across a broader range of scar-mediated VT presentations.





vMap reported as feasible and effective as part of a non-invasive workflow for arrhythmia management when using stereotactic ablative radiotherapy
.png)
A peer-reviewed article published by the Heart Rhythm Society’s journal Heart Rhythm O2 concludes that Vektor’s Computational ECG Mapping System (vMap™) and protocol-based respiratory gating may help facilitate radioablation planning and maintain efficacy during therapy.
The prospective, dual-site study, led by Dr. Gordon Ho, reported that a new non-invasive workflow using vMap™ may help facilitate the radioablation planning workflow. The study concluded that radio-ablation using vMap’s 12-lead ECG mapping and respiratory gated delivery may provide short-term safety and maintain efficacy during therapy in patients with advanced structural heart disease and refractory ventricular tachycardia (VT).
vMap's Role in Optimizing Non-Invasive Radio-Ablation Therapy presented at ACC.21 and THRS 2021.

A dual-center study reported that vMap, as part of a new, non-invasive workflow, improved efficiency and precision for radio-ablation therapy in these study subjects. The study showed that the radio-ablation administered as a part of the enhanced workflow also significantly reduced ICD shocks for patients receiving the therapy. Presented by its lead author Dr. Gordon Ho, at American College of Cardiology 2021 and the Taiwan Heart Rhythm Society 2021 (THRS), the research won first prize in the “Clinical - Ventricular Arrhythmias/Cardiac Implanted Electronic Device” category at THRS. As stated at the ACC and THRS conferences, the study concluded that the simplified, non-invasive workflow, involving vMap, was “Feasible, Efficient, Precise, Effective, [and] Safe”.
The feasibility of using vMap™ in patients after COVID-19 Infection presented at THRS 2021.

COVID-19 is associated with myocardial inflammation which may cause or exacerbate arrhythmias. A study led by Dr. David Krummen was presented at the Taiwan Heart Rhythm Society - Cutting-Edge Care for Heart Rhythm on April 17-18, 2021. The study reports that use of vMap™ computational 12-lead ECG analysis was able to provide arrhythmia insights beyond those available from a standard 12-lead ECG in patients following COVID-19 infection.
Vektor Medical Initiates Clinical Study of vMap™

Vektor Medical, Inc. announced the start of its clinical study to evaluate vMap™. Using patients who have previously undergone a clinically-indicated electrophysiology study and successful ablation, the purpose of this study is to clinically validate the use of vMap™ in providing arrhythmia/pacing hotspots for analysis by a physician. vMap™ has been designed as the next generation in arrhythmia mapping. This non-invasive, rapid technology helps unlock the patient’s ECG, providing information on arrhythmias in all four heart chambers. Vektor’s study will evaluate vMap™ across a variety of atrial and ventricular arrhythmias, including atrial and ventricular fibrillation.
Benefits of Vektor Medical’s vMap® presented at the American Heart Association’s Scientific Sessions 2020

vMap was presented as an innovative technology that may improve “efficiency and precision” in the treatment of complex arrhythmias. All five patients treated with vMap and radio-ablation experienced successful outcomes with a significant reduction in ICD shocks (29±16 to 0.6±0.9) and no adverse events reported.
Vektor’s vMap™ Technology Used Alongside SAbR Planning Software (Varian, Palo Alto, CA) for Non-Invasive Mapping and Radio-ablation of Refractory Ventricular Tachycardia

Life-threatening heart rhythm disorders such as ventricular tachycardia may be eliminated using cutting-edge, non-invasive technology as an alternative to standard invasive catheter ablation procedures, in some cases. In an abstract and associated poster published at the American College of Physicians’ Southern Regional Conference, researchers report successful use of vMap™ technology to guide stereotactic radiotherapy to destroy diseased heart tissue. Researchers report that this non-invasive mapping and ablation has “shown tremendous promise in recent years.” Importantly, for this patient, his “shock burden decreased from 34 ICD shocks in the 6 months preceding … therapy to 0 ICD shocks in the 11 months after treatment. His quality of life improved significantly, and he has resumed an active lifestyle”.
Vektor Medical, Inc. Chosen as a San Diego Venture Group 2020 Cool Company

Vektor has been selected as one of San Diego Venture Group’s ‘Cool Companies’ for 2020. Connect w/ San Diego Venture Group (SDVG) promotes the formation, funding, and development of innovative new ventures in the San Diego community. "We help innovative companies thrive so they can make a meaningful impact on the economic development of the region, and together create a world-class tech ecosystem," Mike Krenn, CEO of Connect. SDVG’s Cool Companies list highlights the fastest-growing, most exciting startups in Southern California.
Computer-Based 12-Lead ECG Analysis Accurately Maps Ventricular Locations

Researchers, in an abstract and associated poster published in the Heart Rhythm Society’s journal Heart Rhythm, find that computer-based analysis of 12-lead ECG accurately mapped 93% of known ventricular locations to the exact ventricular segment. The computer-based analysis required only 3 minutes, plus or minus 2 minutes, per ECG.
Vektor Medical, Inc. Announces the Closing of Its Series Seed Funding Round to Support the Development and U.S. FDA Clearance of vMap™

Vektor Medical, Inc. is pleased to announce its Series Seed financing round is fully subscribed, and the round is officially closed. The investment round will enable Vektor to further invest in investigative research, efforts toward U.S. Food and Drug Administration 510(K) clearance, and development of a commercial product. “With the support of our investors, we are delighted to have successfully closed this funding round in less than two months and with more investor interest than we had room for in the round.” Rob Krummen, COO and General Counsel
12-Lead ECG-Based Mapping Guides Non-Invasive Radiation Therapy

In an abstract published in the American Heart Association’s journal, Circulation, researchers conclude that a case of stereotactic radioablation of ventricular tachycardia illustrates efficacy of non-invasive 12-lead ECG mapping and radioablation in a patient who failed prior invasive VT therapies.
Computerized Mapping Enables New Procedure for Ventricular Fibrillation

A new procedure developed at UC San Diego Health uses targeted ablation to enable treatment for ventricular fibrillation. The procedure is enabled by the use of electrocardiograms to make a computerized map of the heart’s own voltage.
Vektor Medical, Inc. Successfully Closes Its Convertible Note Funding Round

Today, Vektor Medical, Inc. announces the closing of its Convertible Note funding round. The funds will be used to support research relating to and development of a non-invasive computational arrhythmia mapping device. “We’re excited to welcome our investors on board and join Vektor’s journey in creating the next generation of arrhythmia mapping technology. This funding round enables us to build out our team and further research and develop the technology created by our UCSD founders”. Mike Monko, CEO & Co-Founder of Vektor Medical, Inc.
